It is produced by solvary process by using common salt sodium chloride ammonia and limestone. The Solvay process results in soda ash predominantly sodium carbonate Na 2 CO 3 from brine as a source of sodium chloride NaCl and from limestone as a source of calcium carbonate CaCO 3.
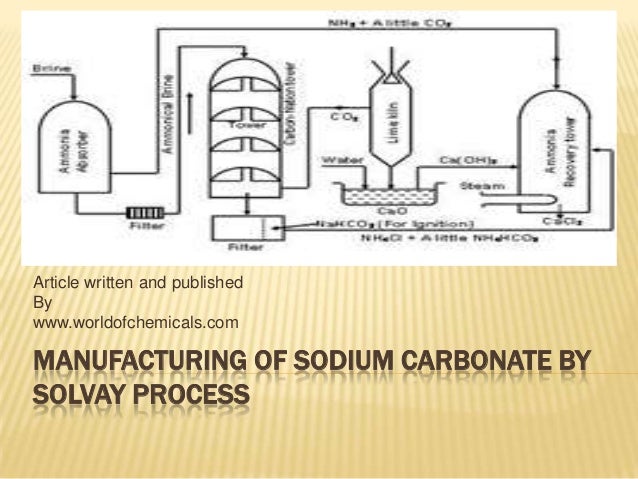
Manufacturing Of Sodium Carbonate Using Solvay Process
8 rows Solvay Process for the Production of Sodium Carbonate Key Concepts.
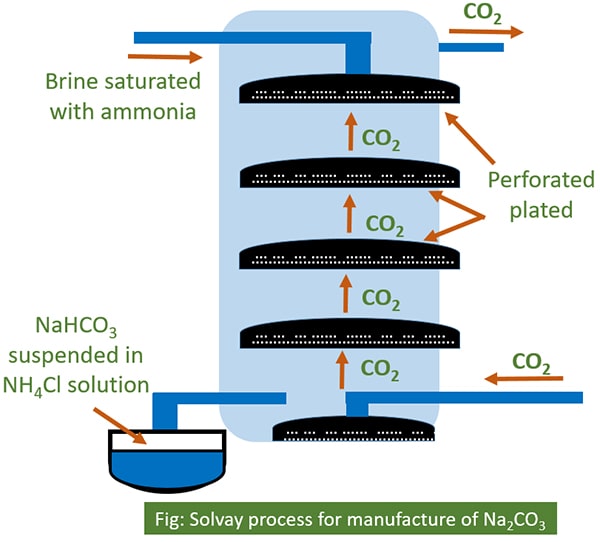
Explain solvay process for manufacture of sodium carbonate. The carbon dioxide is sent back to the Solvay Tower for use in step 3. Include equations in your answer. Brine is saturated with ammonia gas and slowly passed through solvay tower from top to bottom.
Sodium hydrogen carbonate later forms sodium carbonate. Na2C03solution H20gas C02gas 2 NaHC03solid 7 Refined sodium bicarbonate may also be produced from sodium carbonate obtained by other processes such as a sodium carbonate monohydrate process or a sodium sesquicarbonate process these processes generally being supplied with trona or nahcolite minerals. The product of the process anhydrous sodium carbonate is obtained as a fine white powder known as light sodium carbonate.
It has many uses but one of the major notable applications is in making of glass. CaO is formed as a by-product of the thermal decomposition of limestone in the lime kiln. The overall process is.
The Manufacture of Sodium Carbonate the Solvay Process Sodium carbonate Na 2 CO 3 is found naturally or is manufactured from natural salt ie sodium chloride common salt. Solvay Process of sodium carbonate. Sodium carbonate is prepared by passing carbon dioxide through ammonia that gives ammonium carbonate.
Carbon dioxide is recirculated to carbonation tower. Sodium carbonate is now exclusively manufactured by the Solvey process. 2NaHCO3 è Na2 CO3.
Suspended sodium hydrogen carbonate is removed from the carbonating tower and heated at 300oC to produce sodium carbonate. The ammonia-soda process was developed into its modern form by Ernest Solvay during the 1860s. I Outline the chemistry of the production of sodium carbonate in the process shown.
The Solvay process or ammonia-soda process is the major industrial process for the production of sodium carbonate soda ash Na2CO3. 3 marks ii By making specific reference to the diagram justify the requirements for the location of a Solvay process plant. The counter current principle is used in sodium carbonate manufacturing process.
In the reactions which occur sodium hydrogen carbonate is formed which is only very slightly soluble in the presence of sodium ions is almost completely precipitated. Solvay Process as industrial process for obtaining sodium carbonate from limestone ammonia brineThis video is about. Dry sodium bicarbonate is heated in rotary furnace called CALCINER to give anhydrous sodium carbonate or soda ash.
It has an alkaline taste and forms a strongly alkaline water solution. Inside the tower there are a number of mushroom shaped perforated plates. Sodium carbonate Na 2.
The diagram shows part of the Solvay process for producing sodium carbonate. The solvary process centered on a large hollow tower. Making sodium carbonate by the Solvay process involve many reactions but the main parts of the process include purification of the brine formation of the sodium hydrogen carbonate and sodium.
In this process carbon dioxide and ammonia are passed into a cold saturated solution of sodium chloride. Carbon dioxide is sent from bottom to top. Pure sodium carbonate is a white odorless powder that is hygroscopic means it absorbs moisture from air.
Soda ash is a key chemical for producing soap paper making baking soda production and bleaching fabrics and paper. 2NaHCO 3s Na 2 CO 3s H 2 O g CO 2 g Ammonia Recovery. The sodium hydrogencarbonate is heated in rotating ovens at 450 K so that it decomposes to sodium carbonate water and carbon dioxide.
Formation of sodium carbonate. 2 NaCl CaCO 3 Na 2 CO 3 CaCl 2 The actual implementation of this overall reaction is done is four steps. Subsequently ammonium carbonate is converted to ammonium hydrogen carbonate that reacts with sodium chloride to precipitate out sodium hydrogen carbonate.
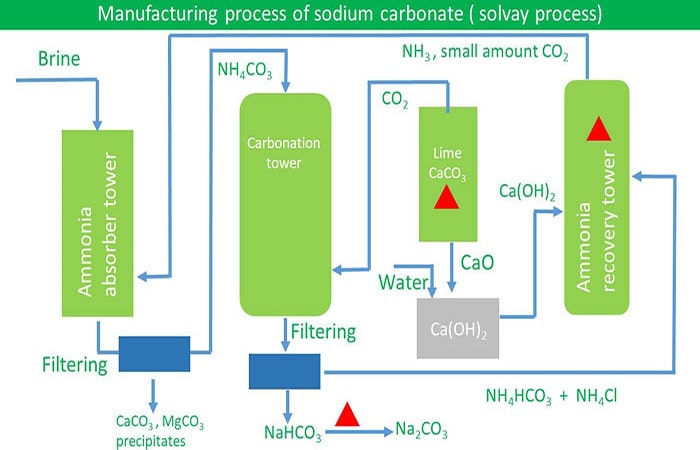
Sodium Carbonate Manufacturing Process Solvay Process
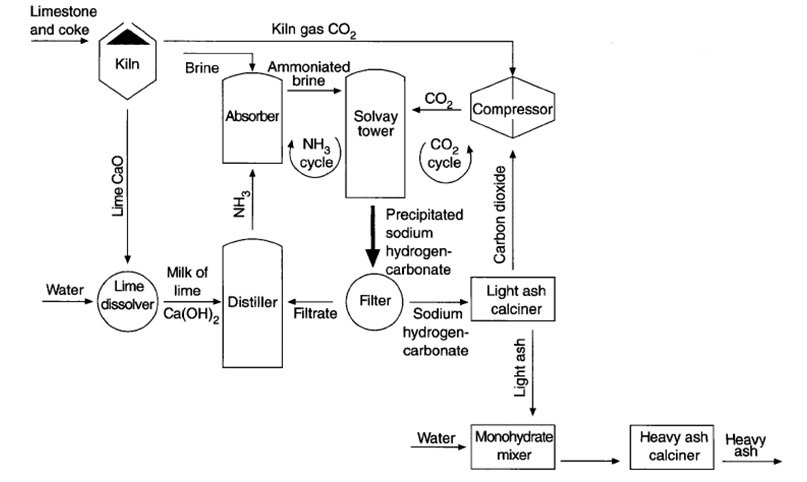
The Steps Used In The Solvay Process Easychem Australia

Sodium Carbonate Manufacturing Process Solvay Process
Https Edu Rsc Org Download Ac 15601
